|
LUNAR ORIGINS
|
People tend to take the Moon for granted. It’s always there, up in the sky - well at least for much of the time! We’ve been there and back. We even brought bits of it back home to analyse – and we’ve not been back in 40 odd years. So what’s not to take for granted? Well, some scientists have been making some pretty well developed arguments that strongly suggest we need to pay more attention to our Moon. In fact, according to them, our scientific understanding of the Solar System has evolved to such a point that we need to go back to the Moon - now - in order to test key aspects of our models of planetary body formation.
As amateur astronomers, we’re well aware of the Moon’s significance in terms of its outsize mass normalised to that of its host planet – Earth; its long-term stabilising influence over the precession of Earth’s axis of rotation; and, thereby, our global climatic zonation.
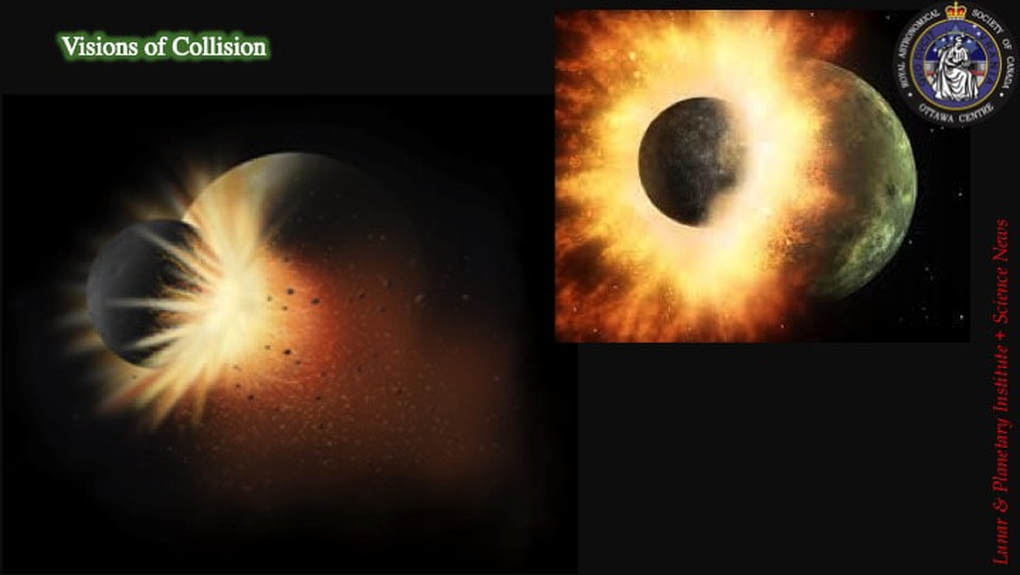
We’re also well aware of the modern scientific consensus regarding both the origins of the Moon during a collision of a Mars-size impactor with the primordial Earth at ~4.6 Ga (first proposed in the mid-1970s), and the subsequent impact activity on the Moon as part of the Solar System-wide Late Heavy Bombardment at ~4.0-3.8 Ga. However, what you may not be aware of is that the modern consensus has been much less of a consensus than you might think, and for quite some time at that! Let’s be clear. Yes, indeed, the Moon probably did arise due to a collision, but what kind of collision? Yes, the surface of the Moon does indeed reflect major bombardment early in the history of the Solar System, but do we really know what that bombardment represents, how it evolved, and what consequences it might have for understanding how our Solar System, our own planet and even the life that covers it evolved? These are two quite separate debates, but both have been quite lively in recent years, which is why I thought it was time to discuss them in a set of two presentations.
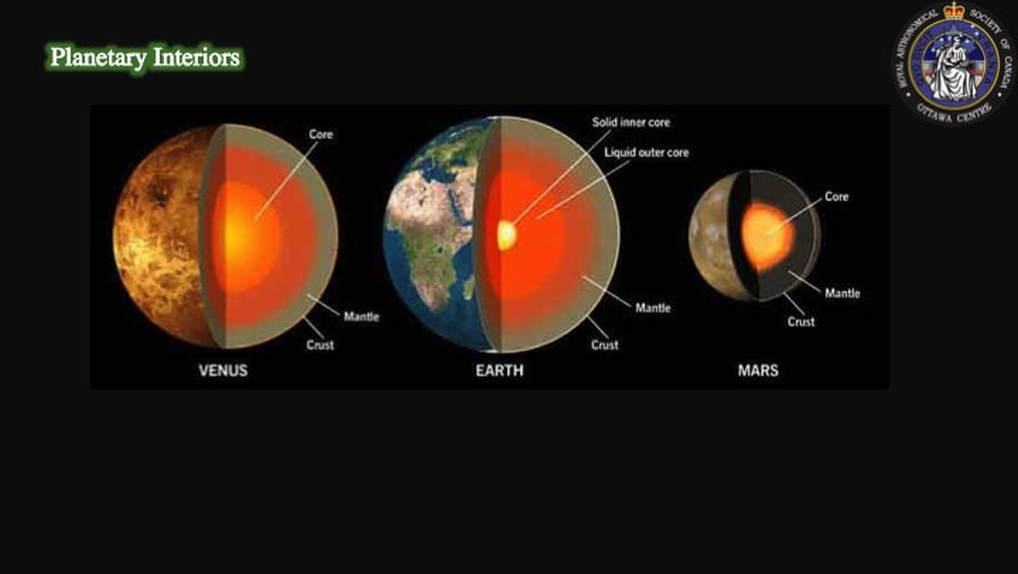
Let’s start here with creating the Moon in the first place. There are two fundamental physical arguments that strongly favour creating the Moon by a major collision early in the history of the Solar System. First, during major planetary collision, the addition of the Fe-Ni core of the impactor to that of the proto-Earth explains the large and small cores of the Earth and Moon, respectively. I have to say I have a bit of a problem with this: how come Venus is Earth’s twin in terms of its internal structure if Earth’s core is the result of an accidental collision? But let’s not get bogged down with details just yet. Let’s just go with the consensus for now …
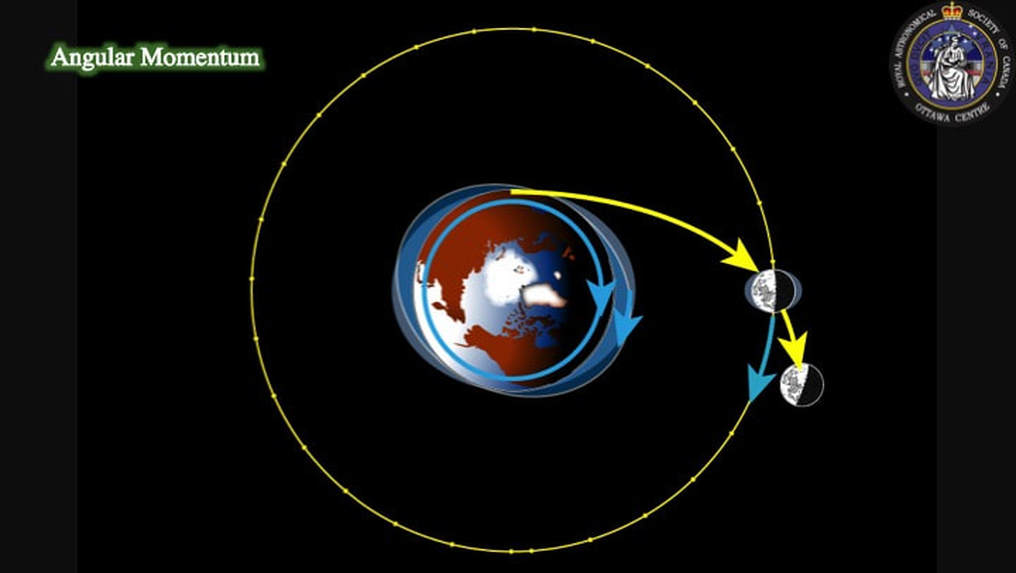
Second, it turns out that it is very difficult to explain the total angular momentum (orbital and rotational characteristics) of the Earth-Moon system without the additional kick that would be provided by a major collision. OK, so what’s the problem? - Chemistry, or more precisely isotopes! The collisional model has given rise to much heated debate stemming from a fundamental problem: when the isotopic compositions of the Moon and Earth were compared, they appeared to be indistinguishable from each other.
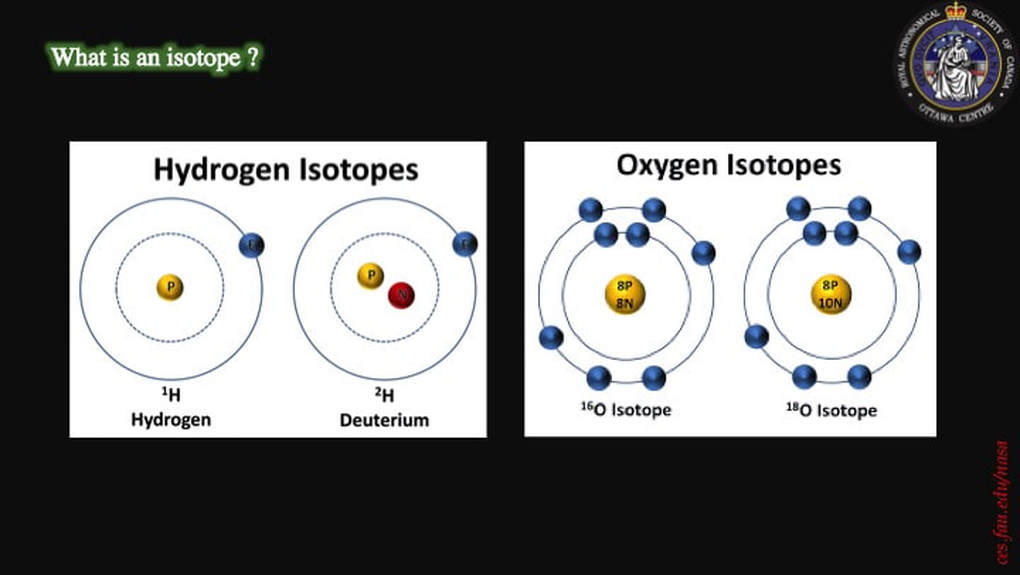
Let’s just clarify what we mean by “isotope”. It’s really very simple : atoms have a nucleus surrounded by electrons. The simplest atom is hydrogen made of a single positively charged proton in the nucleus and a single negatively charged electron whizzing somewhere about the nucleus. Hence the atom is electrically neutral. If we now add an electrically neutral neutron to the nucleus, the atom becomes heavier, but in terms of its electrical – or chemical - properties it behaves just like hydrogen. Hence the term isotope (from the Greek). This heavier version of hydrogen - deuterium - is an isotope of hydrogen. Oxygen is a bit more complicated: it has 8 electrons surrounding the nucleus. In a neutral atom of oxygen, the nucleus always contains 8 positive protons to balance the negative charge of the electrons, plus 8 neutral neutrons. 8 protons plus 8 neutrons gives 16, which is the atomic number of the lightest isotope of oxygen, written as O16. If we now add one or two additional neutrons, we get O17 and O18, heavier isotopes of oxygen that, in terms of their electrical – or chemical – properties, behave just like O16. However, lighter and heavier isotopes e.g. of oxygen can behave differently from each other because of their different masses and weights. For example, at the appropriate temperatures, solid oxygen will melt and eventually form a gas, with the lighter isotopes tending to jump into the gas more easily than the heavier isotopes that tend to be left behind in the liquid or solid state. Technically this is referred to as partitioning. All elements can have isotopes, and behave in much the same way. So now that we know what isotopes are and how they behave, let’s look at the Earth-Moon system.
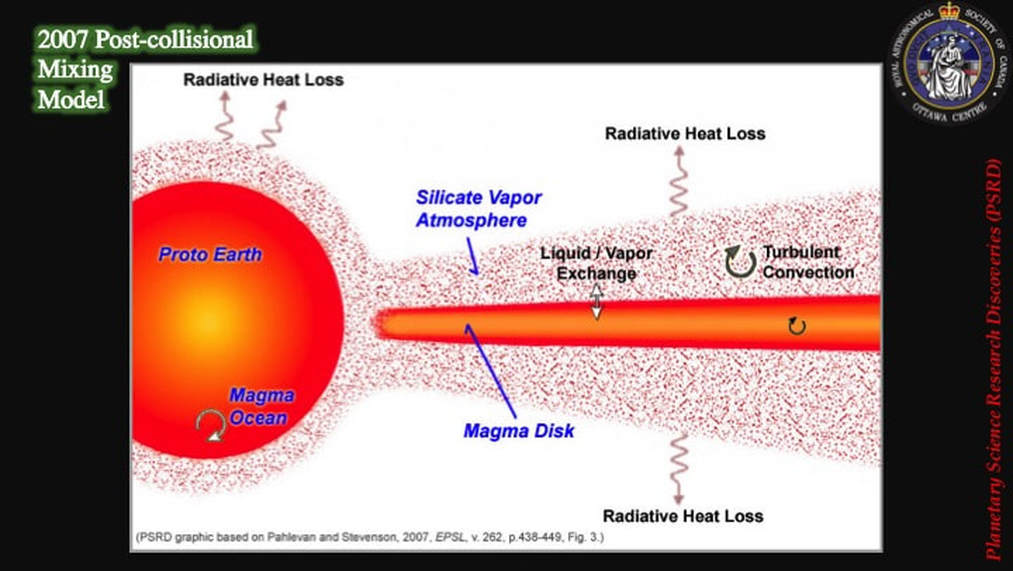
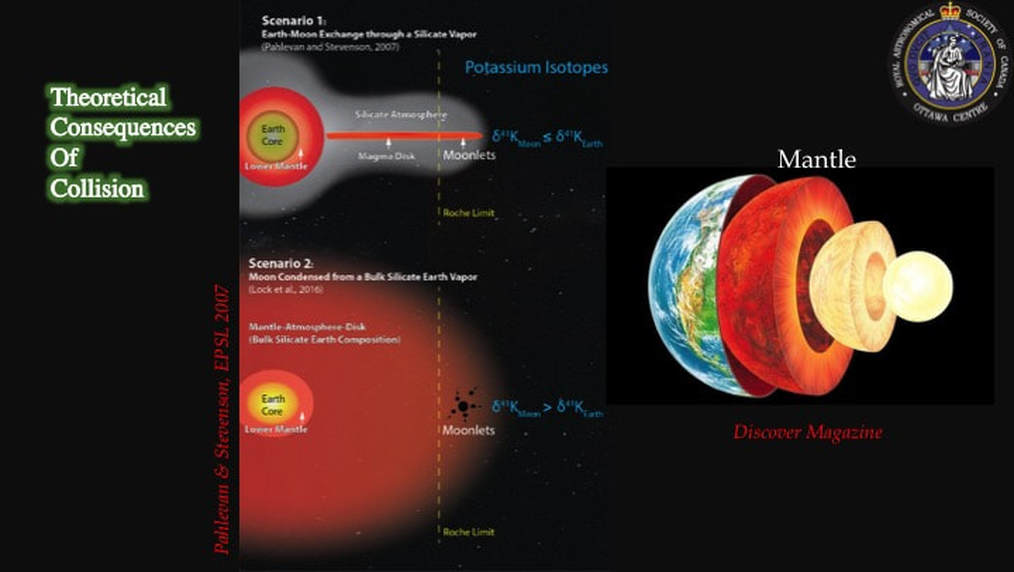
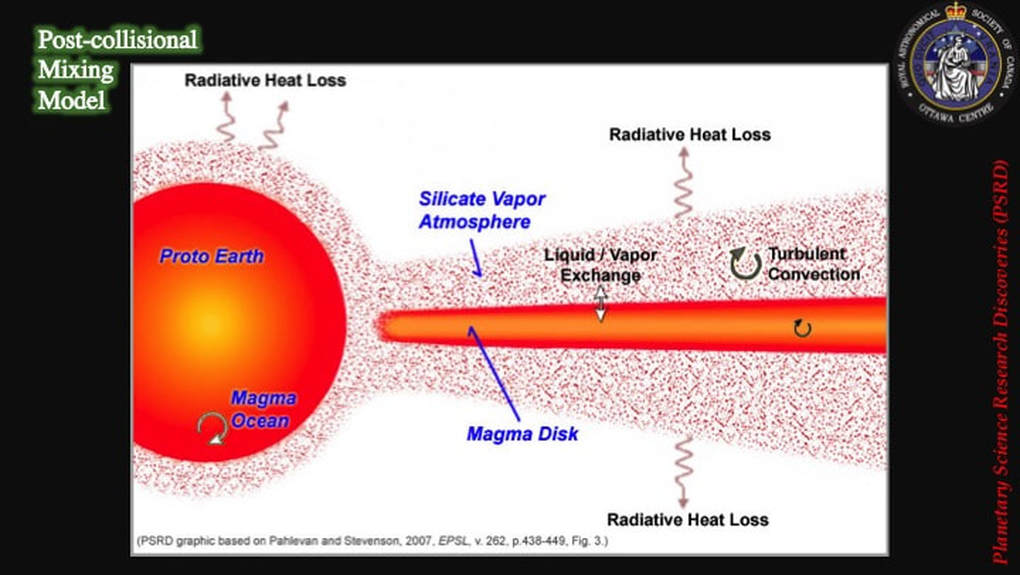
As late as 2012, chemists were bothered by the simple fact that the Earth and the Moon were isotopically identical in terms of their oxygen, titanium, tungsten and chromium contents. Models of what happened after collision, dating from 2007, predicted that a molten proto-Earth would be encircled by a magma disk, itself sandwiched by an envelope of silicate vapour - i.e. vapourised rock. They predicted that partitioning would induce lighter isotopes of an element to leave the magma disk and jump into the silicate vapour more readily than heavier isotopes of the same element. If the vapour was then lost to space, the magma disk would find itself enriched in the heavier isotopes of the element in question, compared to the same magma present in the proto-Earth. When the Moon condensed from the magma disk, it should therefore – theoretically – have been impoverished (depleted) in light isotopes and enriched in heavier isotopes, compared with the same elements on Earth. However, until very recently, this was not the result that was coming out of chemistry labs; the Earth and the Moon were observed to be isotopically identical.
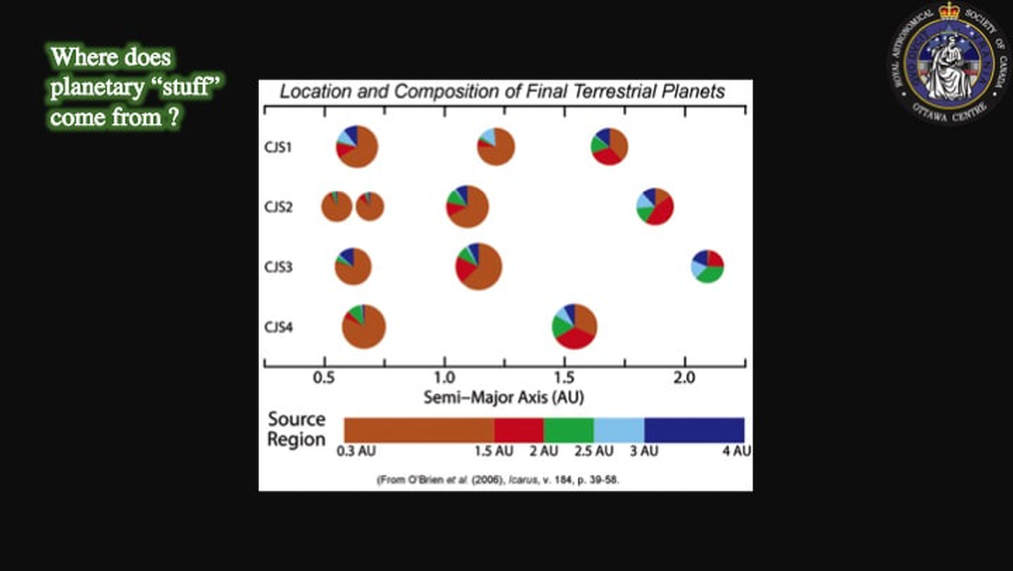
As you can imagine, this created problems for the modellers! In fact, the problem became formally known as the Lunar Conundrum: hence the title of this presentation. Some scientists took this to mean that both the proto-Earth and the impactor were themselves originally isotopically identical, principally because no-one could come up with a viable mechanism to allow the parts two isotopically different bodies to mix thoroughly in the time available for the process. However, this created its own problems. Dynamic models of planetary accretion indicated that planetary bodies are made up of “stuff” sourced from all over the Solar System, but in different proportions from one planetary body to the next. The consequence of this for our story is that it is highly improbable that any two planetary bodies would have the same composition, including isotopically.
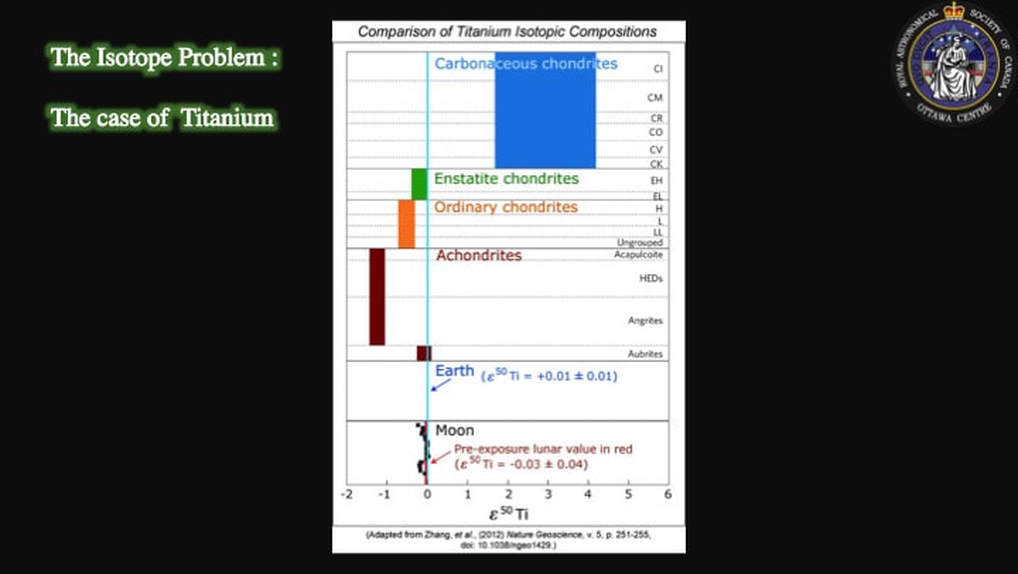
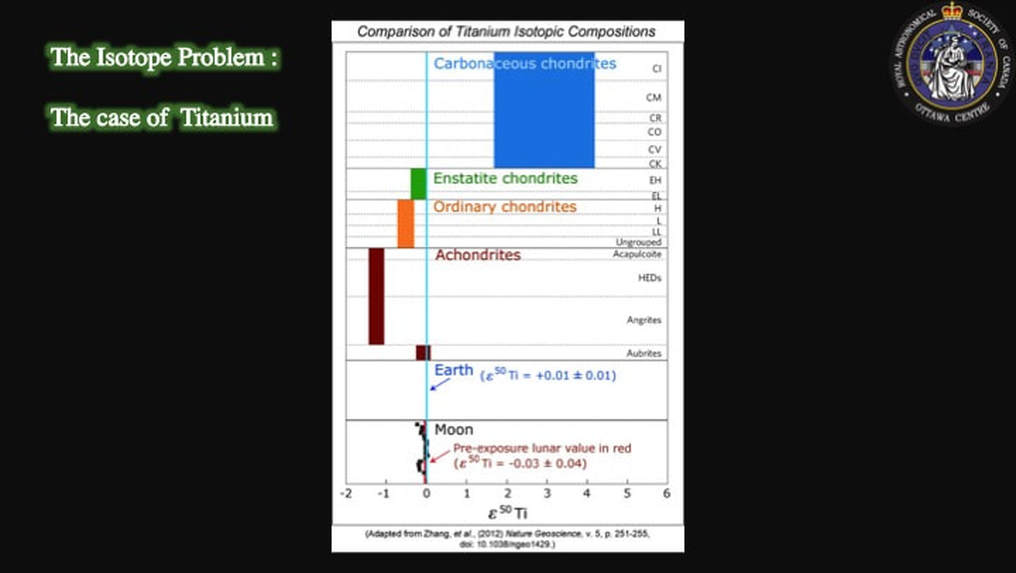
This isn’t just theoretical. When meteorites are measured for their isotopic compositions, they are not only very different from Earth, they are very different from each other. Yet, the Moon (black squares) and the Earth (blue line) are identical, as you can see here in this plot of titanium isotopic compositions. Some scientists went so far as to claim that there was no contribution from an impactor at all - if there even was one - and that the proto-Earth was the sole parent of the Moon; hence the isotopic homogeneity. Remember, we’re not talking 50 years ago. This is a debate that has been on-going over the past decade.
Looking to support the collisional hypothesis, various versions of the physics of the collisional model have recently been proposed to account for the problems posed by the then available chemistry. Some looked to computer modelling to vary the parameters governing the collision. For example, what if we make the impactor way bigger than Mars? What if it was about the same size as the proto-Earth? What if the impact occurred at relatively slow speeds?
Such a catastrophic collision would create a disk with the same composition as the Earth’s mantle (lower right: top right is same as Slide #7), such that the Moon would be Earth-like in composition. This is a neat trick on a computer, but it created a planet Earth that spun 2-2.5 times faster than angular momentum models for the Earth-Moon system predict. So, someone else came up with another computer model that showed how gravitational interaction (resonance) between the early Moon and the Sun could have decreased the angular momemtum of the Earth-Moon system and taken care of this problem.
But now there was a new problem. The very model that took care of the fast-spinning post-collisional Earth also predicted that a small, Mars-sized impactor colliding with a fast-spinning pre-collision Earth could produce all the same results, including the composition of the post-collisional Moon. Why? Because the faster spin of the pre-collisonal Earth would throw Earth material into the disk and throw impactor material out into space. Other scientists picked up on the idea of a high speed, small impactor and their computer models suggested that near-miss or “hit-and-run” scenarios could also fling impactor material into space and produce a similarly isotopically homogeneous Earth-Moon system. Well, while it sounds good - maybe it’s all a bit too good to be true. Computer models can be overly constrained: the variables in the computation have to be just right to get the Goldilocks result the scientists are seeking. But let’s move on …
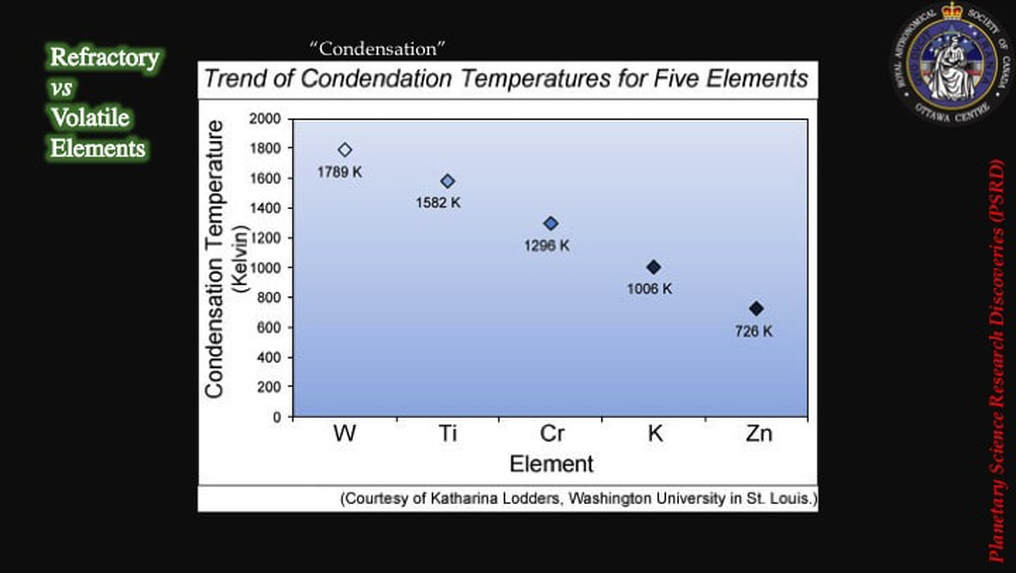
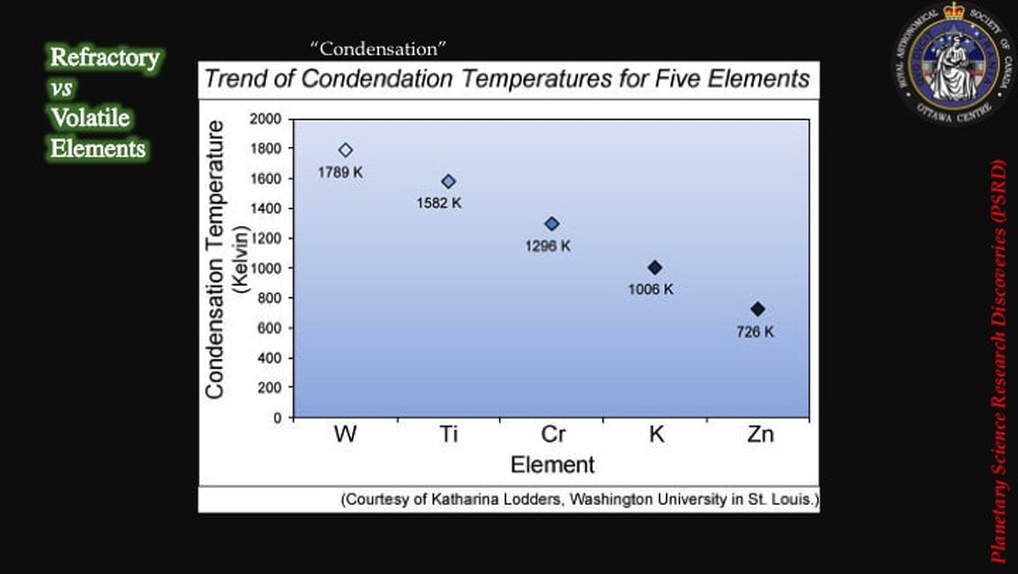
So much for the physics; what about all that chemistry? Well, some chemists had a fundamental re-think. Maybe they were going about things the wrong way. Until 2012, they had assumed that they should be looking for isotopic differences between Earth and Moon materials in refractory elements like Ti (titanium), W (tungsten), or Cr (Chromium), those that don’t easily turn into gas even at elevated temperatures and – therefore – would not easily get homogenised, and hence would preserve the original isotopic signatures of the two colliding bodies.
But in 2013 that idea was apparently shot down by results such as these, that we looked at earlier in this presentation, published in the respected journal Nature Geoscience, and that showed identical isotopic Ti signatures for the Earth (blue line) and the Moon (black squares). The consensus on the collisional origin for the Moon was being seriously questioned.
However, the new computer modelling results had suggested ways of getting rid of the impactor chemical signature: throw it off into space. In effect, this turned the chemistry question on its head: instead of looking for the isotopic signatures of two chemically different colliding bodies in the refractory elements, the issue becomes - where is the evidence for the theoretically predicted isotope partitioning in the post-collisional disk that’s supposed to lead to an enrichment in heavy isotopes in the Moon compared with the Earth?
So it occurred to some scientists - what if they looked at volatile elements, such as Zn (zinc), that turn into a gas at lower temperatures? Would Zn show evidence of isotopic differences between the Earth and its Moon? - evidence that would support the collisional model and the concept of isotopic partitioning in a post-collision disk of vapour and magma!
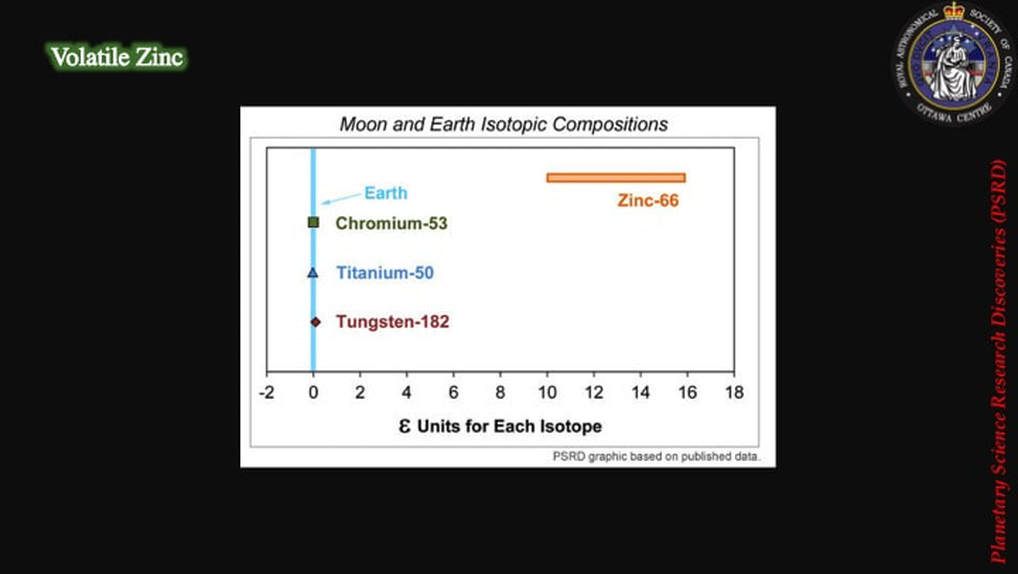
This simple diagram shows that while lunar Cr, Ti and W are isotopically identical to Earth compositions (blue), there’s a lot of isotopic variation in Zn in the Earth-Moon system.
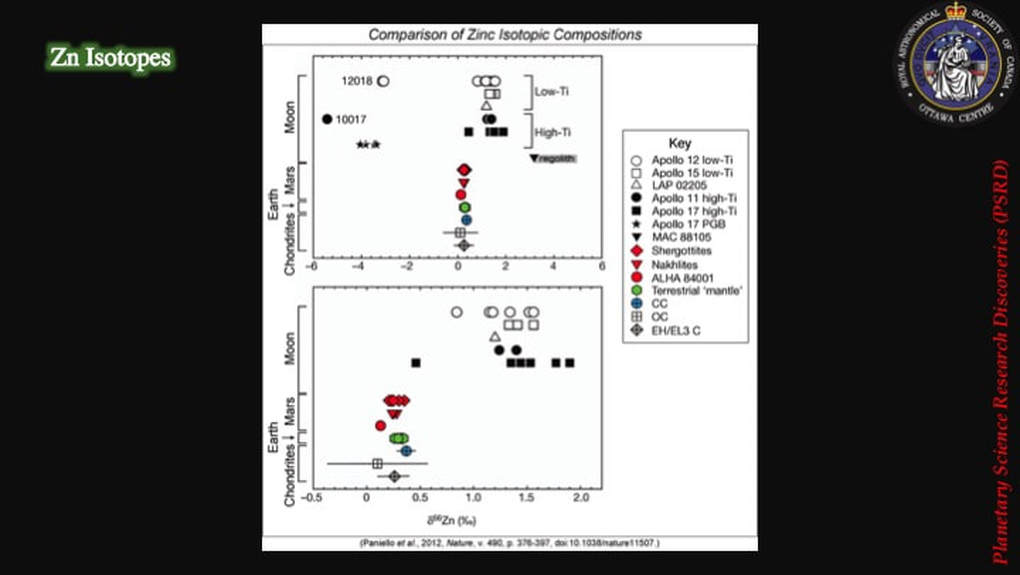
These are the actual isotopic results derived from these analyses. The lower box is just a close-up or detail of the upper box. The data clearly show that the Earth’s mantle (green) and meteorites from across the Solar System (colours) are quite distinct from lunar samples (open and filled black symbols). In addition, most of the lunar samples are isotopically distinctly heavier (they plot to the right side of diagram) than the other materials, interpreted as finally providing evidence for partitioning of heavy isotopes into a post-collisional magma disk from which the Moon was created, and of lighter isotopes jumping preferentially into a gas envelope that was then lost to space. As you can imagine, this made some scientists – those in favour of the collisional hypothesis – very happy.
BTW : No-one has a good explanation for the lunar samples on the left of this plot, samples that are anomalously isotopically lighter than the other Moon rocks, but right now, no-one seems to be worrying about it either !
BTW : No-one has a good explanation for the lunar samples on the left of this plot, samples that are anomalously isotopically lighter than the other Moon rocks, but right now, no-one seems to be worrying about it either !
Meanwhile, back at the lab, other scientists began to wonder if lunar-derived meteorites might have been contaminated during their residence time on Earth prior to discovery. In the case of oxygen isotopes, contamination with terrestrial water (H2O) might mask the original signature. So, in 2013, when Apollo Mission lunar samples were analysed instead of lunar meteorites, a small – but significant – difference was indeed detected. As in the case of Zn, the oxygen in the lunar samples was isotopically heavier than in Earth materials (I haven’t found a diagram of these results yet!).
Well, you might say, that pretty much clinches it. High speed collision of a Mars-size impactor with a fast-spinning proto-Earth throws the impactor material – except for its Fe-Ni core that joins with Earth’s Fe-Ni core – out into space, volatilises much of the proto-Earth’s mantle that forms an isotopically homogeneous disk of melt, solids and vapour from which the Moon condenses, but not before some volatile elements in the disk - like Zn and oxygen - had a chance to partition lighter isotopes into the gas that escaped to space, and heavier isotopes into the remaining melts and solids that ended up forming the Moon. However, the story of the Lunar Conundrum isn’t over yet! In 2014, the researchers who had worked on the oxygen isotopes of the Apollo lunar samples announced that the partitioning of heavy isotopes into the Moon suggested that the Moon might represent a 50:50 mix of Earth and impactor materials, which would seem to take us back to square one !
Well, my take-home message here is somewhat tongue-in-cheek, and it’s this : if you’re a bit confused at the end of all this, you’re not alone, believe me. That’s the scientific method for you! Stay tuned for updates !
Well, you might say, that pretty much clinches it. High speed collision of a Mars-size impactor with a fast-spinning proto-Earth throws the impactor material – except for its Fe-Ni core that joins with Earth’s Fe-Ni core – out into space, volatilises much of the proto-Earth’s mantle that forms an isotopically homogeneous disk of melt, solids and vapour from which the Moon condenses, but not before some volatile elements in the disk - like Zn and oxygen - had a chance to partition lighter isotopes into the gas that escaped to space, and heavier isotopes into the remaining melts and solids that ended up forming the Moon. However, the story of the Lunar Conundrum isn’t over yet! In 2014, the researchers who had worked on the oxygen isotopes of the Apollo lunar samples announced that the partitioning of heavy isotopes into the Moon suggested that the Moon might represent a 50:50 mix of Earth and impactor materials, which would seem to take us back to square one !
Well, my take-home message here is somewhat tongue-in-cheek, and it’s this : if you’re a bit confused at the end of all this, you’re not alone, believe me. That’s the scientific method for you! Stay tuned for updates !
Proudly powered by Weebly